As science advances, Drug Discovery aims to keep fueling new medicines to cure and palliate many ailments and some untreatable diseases that still afflict humanity. But, have you ever wondered just how a new drug or vaccine comes to fruition?
Drug Discovery involves many different phases and processes, from ideation to development to approval. In this article, we’ll explain everything you need to know about the Drug Discovery process, including what it is, some common questions, the four major stages, how Early Drug Discovery, Pre-Clinical, and Clinical Phases work, and how Zebrafish can be used throughout the process.


Index:
What is the Drug Discovery Process?
The Drug Discovery process is a very long process that can take up to 13 years. The Early Drug Discovery process typically starts by screening for potentially active compounds. These compounds must have a therapeutic effect on the intended disease, and after identifying them, testing for safety and effectiveness begins.
Typically, only 1 out of every 5,000 drugs make it to the market approval stage. Moreover, out of 5,000 and 10,000 drug candidates, only 250 make it to preclinical testing. Below, we’ll answer some common questions about the Drug Discovery process.
How Long Does It Take To Bring A New Drug To Market?
As mentioned earlier, the Drug Discovery process can be very grueling. This means it might take more than ten years to develop a new drug. This tremendous amount of time needed can be explained by the fact that an active ingredient has to go through many stages, from discovery to approval. An active compound is said to be a safe drug only after in-depth examination and intensive studies.
How Much Does It Cost To Research And Develop A New Drug?
Taking a drug from discovery to the market is a costly process. The journey of discovering a new drug is estimated to cost around $2.6 billion, as stated by a study performed by the Tufts Center. Beyond just the large investment needed to produce a new drug, the process has also become more cumbersome in recent years. In addition, the post-marketing monitoring and development costs are said to be from $312 million dollars, boosting the entire lifecycle of Research and Development to three billion per drug.
Why It Is Important To Reduce Animal Testing Through The Drug Discovery Process?
Before the drug candidates are tested in any form on humans, their effectiveness and tolerance must be proven in cell cultures and animals. If the candidates turn out to be toxic or harmful, they won't have a chance for further development. During this stage, the use of animal models for testing can be reduced to both follow more ethical guidelines, and to save time and money. There are alternative models, like Zebrafish, that are more cost-effective for researchers, while also respecting 3Rs guidelines. We will expand on this topic further below in the article.
What Are the Four Stages of the Drug Discovery Process?
The Drug Discovery Process involves many different stages and series of actions. Typically, it can be divided into four main stages: Early Drug Discovery, Pre-Clinical Phase, Clinical Phases, and Regulatory Approval. Let’s explore the major steps that are taken in each of these stages to develop a new drug.
_Mesa%20de%20trabajo%201%20copia.jpg?width=606&name=graph(2)_Mesa%20de%20trabajo%201%20copia.jpg)
1. Early Drug Discovery
The Early Drug Discovery Process involves many different actions and testing. Researchers collaborate to identify and optimize potential leads to a specific target. Essentially, the leads must elicit a desirable effect on a specific biological target implicated in a disease, in the hopes of treating it. Research at this point is performed in the laboratory using in silico platforms, biochemical assays, cell cultures, and various animal models. This stage flows through these sub-processes: Target Identification and Validation, High Throughput Screening or High Content Screening, Hit Identification, Assay Development and Screening, Hit-To-Lead (H2L), Lead Generation and Optimization, and In vivo and In vitro Assays. Keep reading on to learn more about each of these different steps within the Early Drug Discovery process.
2. Pre-Clinical Phase
In keeping with the four major phases of the Drug Discovery process, the second stage is the Pre-Clinical Phase. In the Pre-Clinical Phase, the substances identified during Early Drug Discovery are refined, optimized, and extensively tested in a laboratory and in animal or alternative models. The aim is to provide sufficient evidence of safety and efficacy before Clinical Trials in humans can begin, and once this point is assured, it is also useful to calculate the appropriate doses to test in humans. Before the Clinical Trials start it must be ensured that the new substance is available in sufficient quantities during the clinical studies. Since only small quantities were previously required, production now has to be adapted to the significantly higher demand in the Clinical Phase.
Regulatory authorities require preclinical studies before submitting any investigational new drug application to progress to Clinical Phases.
3. Clinical Phases
Clinical Trials are composed of four phases: Phase I, II, III, and IV. We will discuss each of these phases in greater detail further on. Nevertheless, in the first stage, the tolerance and safety of the drug candidate will be tested in a very small group of healthy subjects, usually 20 to 80. Phase I aims to answer the following questions:
_Mesa%20de%20trabajo%201%20copia%204.png?width=200&name=graph(2)_Mesa%20de%20trabajo%201%20copia%204.png)
-05.png?width=200&name=graph(2)-05.png)
-06.png?width=200&name=graph(2)-06.png)
For these studies, the active ingredient must first be manufactured under GMP conditions. That means strictly controlled according to the guidelines of "Good Manufacturing Practice."
After tolerance and effectiveness have been tested in a small group, phases IIa, and IIb are started to examine the effectiveness, tolerability, and dosage in a larger group. For this, the dosage form is first developed.
In phase IIa studies, the therapy concept is primarily checked (proof of concept); in phase IIb studies, the aim is to find the right dose. Phase II studies usually include 100 to 500 adult patients in the study.
In the last phase before a possible approval as a drug, doctors test the drug on thousands of patients to see whether the effectiveness and safety can be confirmed in many different patients. Interactions with other drugs are also tested. Phase II and phase III studies are typically so-called controlled studies: some of the patients receive the new drug, and another group the previous standard drug.
4. Regulatory Approval
When an active substance completes the Clinical Trials, the data is then collected and analyzed. Then, it can be submitted to the appropriate authorities for review. Before a drug or vaccine can be sold, approval from a national regulatory authority or centralized process is required. Ultimately, only one of a large number of compounds tested makes it through the process of clinical study phases and regulatory tests. Therefore, only one compound is approved as a drug or vaccine.
Post-Market Monitoring
Phase IV studies (also known as Post-Marketing Surveillance Trials) take place after receiving marketing authorization from the authorities. More comprehensive data can be gathered regarding the effectiveness and safety of the new drug. A larger number of patients taking the drug provides much data as well as compares against treatments that are already available. These studies are designed to assess the long-term effects of a drug. In this way, adverse events can be recorded and avoided.
The Early Drug Discovery and Pre-Clinical Phase, Step-by-Step
Now, let’s get more in-depth into the different processes that occur during the Early Drug Discovery phase of drug development. We’ll review Target Identification, High Throughput Screening, and High Content Screening, Hit Identification, Assay Development, Hit-To-Lead, Lead Generation, and In vivo and/or In vitro assays.
.jpg?width=617&name=graph(2.2).jpg)
Target Identification and Validation
One of the key factors in designing a good drug is having a crystal clear understanding of the pathogenesis of a disease. A suitable biological target is said to be “druggable” when a therapeutic molecule, called a “hit”, can modify its biological activity. Potential targets may be discovered by sourcing available databases and researching public literature. Once a target is identified, researchers validate their suitability for drug development before going through the screening process to identify the hits.
High Throughput Screening
High Throughput Screening (HTS) and High Content Screening (HCS) are often the most important steps toward discovering a drug. Once a potential target has been identified and validated, the starting point can be detected by screening a large number of molecules that can interact with it. An assay must be developed ad hoc, meaning providing data about the effectiveness and selectivity of the molecule. In addition, it has to withstand the fast and reliable testing requirements. HCS can offer richer data than HTS.
Hit Identification and Discovery
To identify a hit molecule, various methods can be used. The hit is defined as a molecule that must interact with the previously mentioned target to result in a desired therapeutic effect. High Content Screening, phenotypic screening, fragment-based screening, structure-based screening, and virtual screening are examples from a large list of strategies used to discover hits.
Assay Development and Screening
High Content Screening (HCS) is based on the novel approach of taking a large number of images using high throughput microscopy. In vitro cell models including image-based cellular assays and even zebrafish embryos (qualified as in vitro) play a central role in the application of HCS in research for new active ingredients. HCS is used for secondary substance screening, lead optimization, substance profiling, identification and validation of effective targets, investigations in the area of ADME (absorption, distribution, metabolism, and excretion), and for toxicity studies in preclinical research.
Hit-To-Lead (H2L)
As we mentioned earlier, a hit is a term used to describe a chemical compound that has a desired therapeutic effect at a known target molecule. Similarly, the lead is the product of the screening process, which can be used in advanced stages.
The Hit-To-Lead (H2L) stage is one of the most essential stages in early Drug Discovery. The main aim of the H2L is to find the appropriate leads to move along the pathway to a final clinically active drug. Researchers refine the initial compounds using different screening methods, such as High-Throughput Screening, affinity selection of large chemical libraries, fragment-based techniques, and target-focused libraries. The screening process aims to reduce the number of these compounds into less and more qualified leads using in vitro and computer-based approaches. The lead properties must be adequate to examine their efficacy in any in vivo models.
Lead Generation and Optimization
Once the lead is generated in the Hit-To-Lead process, the lead optimization will commence. Lead optimization aims to improve the most promising compound products to enhance effectiveness, lower toxicity, or increase absorption.
In Vivo and In Vitro Assays
At the end of the early Drug Discovery stages, the resultant potential compounds must be tested under conditions similar to living cell conditions. Here comes the role of in vivo or in vitro testing. These testing assays are used to test the safety and the potentially toxic effects of a compound using animal models, alternative models, like Zebrafish, or cell cultures.
Clinical Phases and Regulatory Approval: Everything You Need to Know
After the Early Drug Discovery and Pre-Clinical Phase takes place, then the Clinical Phases commence. Let’s explore the four main processes that occur during the Clinical Trials and Regulatory Approval Phase. +
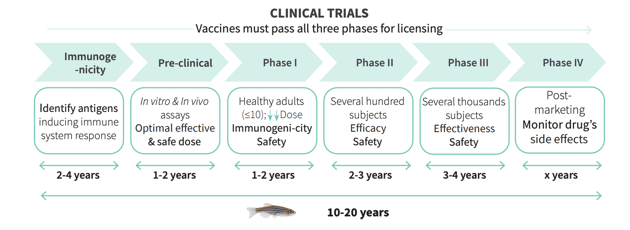
Phases I - III Trials
A clinical study typically involves examining volunteers to test new ways to prevent, and treat diseases. In these controlled studies, vaccines and drugs are tested for their effectiveness and tolerability. Active ingredients have to go through 3 Clinical Phases.
In Phase II clinical studies, the effect is tested on people suffering from a specific disease. Up to 500 patients will take part in the Phase II studies. In this phase, not only the effectiveness and tolerability of the drug should be checked, but also the appropriate dosage should be determined.
In Phase II clinical studies, the effect is tested on people suffering from a specific disease. Up to 500 patients will take part in the Phase II studies. In this phase, not only the effectiveness and tolerability of the drug should be checked, but also the appropriate dosage should be determined.
If the drug has successfully passed Phase II, it will be further investigated in a larger number of patients in Phase III clinical studies. The aim is to show that the drug offers the desired effectiveness in many patients. Rare side effects can also be discovered during this phase.
If Phase III is successful, the manufacturer can apply for approval for the drug - that is, official permission to put the drug on the market.
Pharmacokinetics and Pharmacodynamics Analysis
Following the administration of a drug in an in vivo experiment, drug dynamics data within the body will be derived and described. This analysis is known as pharmacokinetics (PK) and Pharmacodynamics (PD) analysis. This process is very important because it enables the determination of dosing and regimes.
Bioanalytical Method Validation
The validation of bioanalytical methods involves quantitative determinations of the corresponding analyte in biological matrices such as urine, saliva, blood, etc. The validation of such quantitative determinations of the analyte e.g. drugs in development or their metabolic products or biomarkers is crucial for the successful implementation of pre-clinical and subsequent clinical pharmacological studies. Validated methods provide important information regarding the safety and efficacy of the relevant drug under development.
Regulatory Approval and Post Approval
When the development of a drug is almost completed, the approval documentation is drawn up and submitted to the competent authority. This procedure is very time-consuming because all results of the drug testing have to be presented in extensive documents. The approval authority checks all data and decides on approval. The essential prerequisites for approval of a drug are adequate pharmaceutical quality, therapeutic effectiveness, and safety. To do this, the drug must have a favorable risk-benefit ratio, which means that the desired drug effect should be accompanied by as few and harmless side effects as possible. The risk-benefit ratio is the most important approval criterion that is weighed up by the responsible authorities. Even after approval has been granted, the risk-benefit ratio should be continuously monitored. If it changes, this has an impact on the approval. In the worst-case scenario, the drug even has to be withdrawn from the market.
In the course of the authorization process, a very important document is also produced: the “Summary of Product Characteristics.” This document contains all of the important information for patients about the area of application, contraindications, dosage, interactions, and side effects.
FDA Regulatory Approval
The FDA is a federal agency of the United States Department of Health and Human Services. It is responsible for protecting and promoting public health. To achieve this, the FDA controls the safety and effectiveness of all drugs.
For the FDA to approve a drug, it means that the data regarding the drug’s efficacy and safety must be reviewed by the CDER (Center for Drug Evaluation and Research). Moreover, the drug must be proven to provide more benefits than any known or potential risks. The FDA regulatory approval process goes within a very strict-structured process that includes the analysis of the target condition and available treatments, assessment of benefits and risks from clinical data, and strategies for managing risks.
The Benefits of Zebrafish for Ethical and Cost-Effective Drug Discovery Process
In the last few decades, new alternative models have become available to replace traditional animal models. One of the most beneficial alternative models is Zebrafish. Zebrafish embryos and larvae are completely transparent. Thus, by using just microscopes, researchers can look into the internal bodies of the animals without harming or injuring the embryos. Zebrafish are a strong alternative model for Drug Discovery for many reasons including the following:
- Zebrafish can be grown very easily
- They can be housed in large groups
- They can produce 200-300 eggs per fish
- The embryos can hatch two days after fertilization; all the vital organs of the fish will mature five days later and will have taken up their function.
- In just two to three months later, the young fish will reach sexual maturity to be capable of breeding new offspring.
Keep reading to discover more benefits of Zebrafish, including their adherence to 3R regulations, and how they function as an alternative model.
_Mesa%20de%20trabajo%201%20copia%202-1.jpg?width=620&name=graph(2)_Mesa%20de%20trabajo%201%20copia%202-1.jpg)
How Zebrafish Fall Under the 3Rs
The 3Rs – Replacement, Reduction, and Refinement – are embedded into the legislation and guidelines governing the ethics of animal use in experiments. The Zebrafish is a great option for following the 3Rs model.
Zebrafish embryos and larvae are used in the research instead of rodents because apart from their high genetic homology, they are not considered an in vivo experiment until they are 5 days post-fertilization.
The sample size must be large enough to give sufficient results; the Zebrafish can produce 200-300 eggs per fish and HCS assays can be performed.
Are the least invasive methods used? The Zebrafish embryos are transparent, so they can be monitored and examined harmlessly.
Why Zebrafish Are a Good Alternative Animal Model
Zebrafish grow incredibly fast, are small yet strong, and have many organs and genes in common with humans. Scientifically and genetically speaking, 1990s studies have shown that 70% of Zebrafish genes are found in human cells. Moreover, 84% of disease gene homologs are shared between humans and Zebrafish. This is what makes the Zebrafish platform an extremely ideal alternative for preclinical research involving human diseases, including gene silencing and toxicity assays.
Conclusion
By now, you are surely more familiar with the entire Drug Discovery process. From Early Drug Discovery to Pre-Clinical Trials, Clinical Trials, and Regulatory Approval, the Drug Discovery process is a long, but necessary system that helps to improve lives and advance science. Fortunately, with new alternative models, this process can be more cost-effective and shortened while also adhering to more ethical practices.




