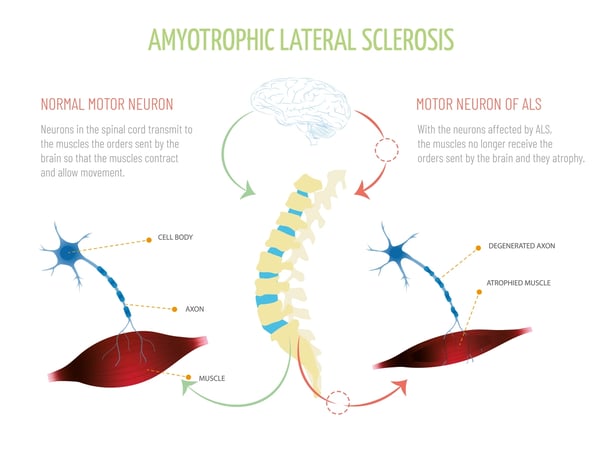
Amyotrophic Lateral Sclerosis (ALS) is a fatal neurodegenerative disease characterized by the progressive loss of motor neurons. These neurons control voluntary muscles, the loss of the motor neurons causes muscle weakness, first in the limbs, progressing to other muscles, until the capacity to fulfill basic tasks such as: move, speak, eat, and finally breathe is lost, causing the patient’s death.
ALS is the third most common neurodegenerative disease after Alzheimer’s and Parkinson’s diseases, affecting around 400.000 people worldwide (prevalence of 4.5 people per 100,000).
This makes ALS a priority for the scientific field to find the physiological and molecular cause of the disease and to hopefully develop effective and safe treatments that can hopefully lead to a cure.

What is ALS?
As mentioned, the motor neuron loss caused by ALS affects the brain and spinal cord nerve cells causing a loss of mobility, speech, the ability to eat, and finally the ability to breathe and, consequently, the death of the patient. Besides, half of the ALS patients present thinking and behavioral difficulties and 15% of them develop dementia.
This disease is also sometimes referred to as Lou Gehrig’s disease, after the famous baseball player who was diagnosed with ALS at 36 and died from it two years later.
Whilst 5-10% of cases of ALS are hereditary (familial ALS), the remaining 90% (sporadic ALS), show no evidence of family history. Genetic studies revealed that around 10-15% of ALS patients have gene variants associated with the onset of the disease. However, ALS is a complex disease as genetic variants have a small impact on the predisposition of disease development and no clear genetic susceptibility has been found. According to environmental factors, some have been related to the illness, including some chemicals such as lead, tobacco, alcohol, or some pesticides; exposure to electromagnetic fields, or doing military service, maybe due to exposure to tobacco and alcohol.
According to ALS epidemiology:
- More than 5,000 people per year are diagnosed with ALS.
- Someone is diagnosed with or dies from ALS every 90 minutes.
- The average life expectancy from the onset of symptoms is 2-5 years.
Children whose parents have familial ALS (genetic dependent) have a predisposition between 20 to 80% chance of developing the disease.
Current ALS treatments
Even though there is no cure, there are four FDA-approved drugs on the US market that can be used to treat ALS: Riluzole, Edaravone, and Dextromethorphan/Quinidine. However, these treatments barely improve the condition and survival of the patients since they only treat the symptomatology of the disease.
Therefore, new specific treatments for ALS are needed but the sky-high costs of around $2 billion and the risk involved in the drug development process confirm the need to use a cost-effective and successful model to investigate this disease and test potential new drugs.
The use of zebrafish in ALS testing
Aside from the genetic link, the cause of ALS in 90% of people who get the disease sporadically is undetermined. No clear genetic or environmental predisposing factors are found in patients, revealing that ALS is, probably, a complex disease developed by the combination of several physiological events produced by both genetic and environmental factors.
Nevertheless, there are several genetic and pharmacological animal models that mimic the pathophysiology of the disease. As of today, some of the best-known ALS models in mice are SOD1, TDP-43, FUS-43 transgenic model, FUS transgenic model and C9orf72 repeat expansion model.
The use of alternative models is gaining more and more potential, even regulatory agencies are persuading and looking for ways to reduce the use of mammals such as small rodents like mice and translating this preclinical research to NAMs such as zebrafish. Mice models are costly, time-consuming, and present high ethical concerns. These are important disadvantages for the Drug Discovery process, where hundreds of candidate compounds need to be tested. In this sense, zebrafish, a small-scale tropical fish, have been implemented as one of the best NAMs for basic research in Drug Discovery and Development. They present several advantages compared to other models including a fast reproduction cycle, with the capacity to generate hundreds of embryos per day, allowing fast and cost-effective tests in days and weeks, while on mammals the same process can take up to several months. This can speed up the study process and allow scientists to discard ineffective compounds and saving valuable research time and resources.
Zebrafish have a remarkable similarity to human genetics and also have an almost interchangeable nervous system and brain function to humans, plus Blood Brain Barrier.
It is also a favorable model for ALS investigation, due to its vast feasibility for genetic modification, enabling the production of genetic disease models and other manipulations to study in more detail the physiology of the disease. Furthermore, it is transparent in the embryonic and larval stages, allowing direct in vivo visualization of internal organs, including neuron visualization.
Concerning the ethical point of view, the fast growth of the zebrafish makes it possible to perform the assays on the embryonic stage, as most organs are functional by 5 days post-fertilization (dpf), when still not considered experimental animals and are under the 3Rs perspective.
Genetic ALS models generated for mice have been also successfully developed in zebrafish, replicating the pathophysiological conditions seen in patients. All these factors highlight that the zebrafish model is truly a useful and valuable NAM for basic research or Drug Discovery and Development, that is aligned with the 3Rs Principles (Replacement, Reduction, and Refinement) for the responsible use of animals for research purposes.
The practicality and adequacy of the zebrafish model for neurodegenerative diseases research can be exemplified by Biobide, which possesses different ALS zebrafish models such as:
- SOD1: zebrafish model that carries loss of function mutations in the human superoxide dismutase 1 (SOD1) gene, which is associated with a subset of familial ALS are among the most widely used ALS models. These fish exhibit motor neuron degeneration, muscle weakness, and progressive paralysis like human ALS.
- C9orf72: A hexanucleotide repeat expansion in the C9orf72 gene is the most common genetic cause of ALS and frontotemporal dementia (FTD). Zebrafish models with expanded C9orf72 repeats have been developed, exhibiting motor neuron degeneration and behavioral abnormalities.

These models implemented in zebrafish allow us to test in High Content Screening (HCS) platforms making this method a cost-effective alternative.
Conclusion
ALS is a major medical concern as it is a fatal progressive neurodegenerative disease, being the third most common, behind Alzheimer’s Disease and Parkinson’s Disease. Besides there is no cure for this illness, the currently available treatments are not effective as target only the symptoms.
Therefore, there is an urgent need to elucidate the pathophysiological basis of the disease and to develope new effective treatments. In this sense, zebrafish ALS models have been postulated as valuable NAMs for basic research and the Drug Discovery and Development process in the search of better treatment options and potential cures for ALS.
The Zebrafish model is becoming each and every day a more accepted and validated option for research, being Biobide a pioneer in this field offering diseases models for as ALS in zebrafish, foreseeing the necessity of fastening the Drug Discovery process for neurodegenerative diseases and, in particular, ALS. Specifically, Biobide is offering to screen Drug candidates for toxicity and efficacy for enhancing research on this pathology for safer and more effective Drug candidates in a fast and cost-effective manner
Sources
- https://www.mayoclinic.org/diseases-conditions/amyotrophic-lateral-sclerosis/symptoms-causes/syc-20354022
- https://encyclopedia.pub/entry/62
- https://www.hopkinsmedicine.org/health/conditions-and-diseases/amyotrophic-lateral-sclerosis-als
- Auderlan M. Gois, et al. Brain Research Bulletin, 159, 32-43, 2020. (https://www.sciencedirect.com/science/article/pii/S0361923019308810?via%3Dihub)





