Zebrafish have traditionally been used for studying the developmental phases of vertebrates. There are many reasons this animal model is used for these important studies, like its genetic homology to humans and the anatomical and functional similarities of its organs which are transparent and visible during the entire formation process.
Another advantage of Zebrafish research is the fact that these developmental stages occur in a matter of only hours and take place externally, implying that the mothers are spared from any sacrifices. By 48 hours post fertilization (hpf) most of the main organs of the fish have been formed. According to the EU Directive 2010/63/EU, Zebrafish fish are considered an in vitro model before 5 days of fertilization.
In this article, we will cover Zebrafish larvae and explain how researchers can take advantage of this time-frame to study developmental toxicity phenomena in an animal model which falls under the 3Rs principle.

Zebrafish and Toxicity
Toxicity is perhaps the most important factor to bear in mind during the drug development process of a new molecular entity. Any compound released to the environment or eventually given as a drug for any potential treatment must fulfill the safety criteria. This is why toxicity must be the first hurdle to pass before engaging into functional assays of a particular compound. Something unique for the Zebrafish animal model and in contrast with rodent models, is to be able to expose an early toxicity profile in a whole complex system and yet allow a higher throughput of screening than in mammals. Furthermore, High Content Screening can also be applied using images taken from the transparent embryos.
Zebrafish embryos develop inside eggs and aleatory hatching occurs between 48 and 72 hpf. Regardless the embryos have left the chorion or not during this time, after 72 hpf they are called larvae.
Benefits of Zebrafish Larvae for Research
Now we will focus on the main benefits of Zebrafish larvae for research, taking into account the developmental time and the toxicity assays that can be performed.
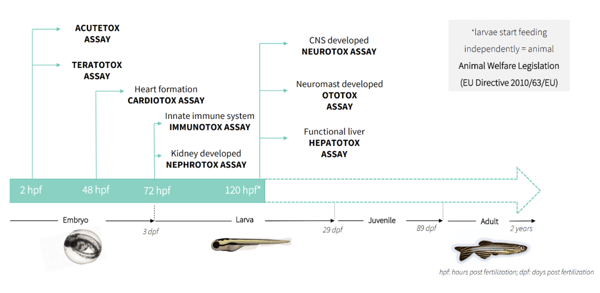
Acute toxicity can be checked according to the OECD 236 Guideline (Fish Embryo Acute Toxicity Test). This is a very convenient, cost-effective, and rapid test that can be performed before any other specific toxicity. A more in-depth general toxicity assay at this early stage is the one concerning reproductive toxicity to see potential birth defects in the form of teratogenic formations. The international ICH S5(R3) guideline states the need to assess chemical safety before any compound enters clinical trials. This guideline moreover remarks data generated from qualified alternative assays conducted alone or in conjunction with one or more in vivo studies that can be utilized to support hazard identification and risk assessment under some circumstances.
By the second day of life, Zebrafish have already developed their heart and this facilitates a perfect model to see potential arrythmias or any heart malformations that can be exposed in a cardiotoxicity model. The heart along with the liver and the central nervous system are the most important checkpoints for any safety assessment.
On the third day of development, the embryos become larvae and two are the main toxicity assays that can be seen at this stage. On one hand, their kidneys are fully functional at 72 hpf and therefore nephrotoxicity studies can be carried out. On the other hand, the immune systems can be analyzed by means of quantification of immune cells thanks to the transparency of these animals.
The last organs to be developed in Zebrafish include the ear, the liver and the central nervous system. These are very important organs which become functional around the fifth day of development.
The hepatotoxicity can be assessed in wild type or transgenic Zebrafish model which highlights the liver with a fluorescent protein. CYP genes are grouped in the same 18 families found in mammals and so any alteration in their expression can expose a breach in metabolic activity. Other problems such as steatosis or oxidative stress can also be seen with this model.
Last but not least, the brain is a very important organ when it comes to toxicity. Several locomotor activity behavioral assays can be employed for the correct function of the brain and the Central Nervous System (CNS) in general. When this organ is altered in any way, Zebrafish larvae respond differently to many types of stimuli such as reacting to a physical contact or showing hyperactivity in the context of light and darkness. All these behaviors can be traced and analyzed with automated image analysis, making this animal model a perfect fit to study CNS alterations.
Finally, apart from adding value to the safety side, these fish are also very useful in disease generation models, creating new genetic lines for target validation or efficacy assays. CRISPR is the current state of the art technology in gene editing and can facilitate the generation of these transgenics. Another alternative to gene editing in Zebrafish is the use of morpholinos, quicker technology that employs small oligopeptides to block the expression of genes.
All the above assays are critical for the safety assessment of new compounds and make the Zebrafish model an essential tool within the research and development of new drugs.





