As the liver is the most important organ in Drug metabolism, one of the major causes of drug attrition in the process of Drug discovery is Drug Induced Liver Injury (DILI). The low correlation of the in vitro cytotoxicity studies with human hepatotoxicity, especially in the early stages of Drug development, makes human hepatotoxicity difficult to predict. Furthermore, traditional in vivo rodent studies commonly used are associated with ethical, economical, and scientific issues.
Smaller, lower-order vertebrates, such as the zebrafish, have similar molecular and cellular processes that can accurately model human physiology. The zebrafish model has been widely used in the field of toxicology and developmental biology and is proposed as a model for predicting hepatotoxic agents due to the functional similarities of the zebrafish liver with the mammalian liver. In addition, zebrafish shows great potential to be used in the early stages of discovery due to its properties that include transparency, external fertilization and fast organogenesis, high genetic similarity with other vertebrates, cost-effectiveness, and the possibility to generate transgenic lines targeting specific organs and pathways, also liver and metabolic pathways. This makes zebrafish an in vivo screening model close to the mammalian model with the advantages of in vitro due to being able to use in vitro screening tools.
Hepatotoxicity
The vital organ toxicities, especially hepatotoxicity, cardiotoxicity, pulmonary toxicity, and neurotoxicity, are of major concern for their high Drug attrition. During the last decades, organ-specific toxicity has been the main concern for Drug withdrawal from the market. The US FDA approved a total of 496 new Drugs in the last two decades and among them, 35 were withdrawn from the market because of organ-specific toxicities, and 107 were reported to be associated with post-market adverse drug events.
The liver is the principal organ responsible for the metabolism of xenobiotics and significant functions such as the emulsification of fats, digestion, detoxification, and protein synthesis. The metabolism of xenobiotics includes biotransformation for the inactivation of toxic compounds, but this biotransformation may also produce toxic reactive metabolites, leading to xenobiotic-induced liver injury. The main problem is that liver toxicity is usually identified in the late stages of Drug development or after a new Drug has already been released to the market (post-marketing).
Hepatotoxins are related to a variety of mechanisms causing liver damage and leading to different grades of liver conditions such as zonal necrosis, hepatitis, cholestasis, steatosis, granuloma, and even neoplasm. When focusing on Drugs such as xenobiotics, steatosis (an increase in cellular lipid content) is the most prevalent phenotype and the leading diagnosis in DILI, associated with the requirement for liver transplantation and with fatal outcomes.
In vitro assays are very effective tools for detecting particular tissue-specific toxicity phenotypes, but it is difficult to detect toxicity based on complex mechanisms involving multiple organs and tissues. Drug-Induced Liver Injury is poorly predicted by single-cell-based assays, probably because of the lack of physiological interactions with other cells within the liver. A challenge in toxicology is to predict the hepatotoxic potential of compounds to which humans are exposed, preferably through New Alternative Methodologies (NAMs), since traditional in vivo rodent studies are associated with ethical, economical, and scientific issues. Therefore, models with higher throughput and improved for predicting human DILI are needed.
Zebrafish as Drug-induced hepatotoxicity model
Zebrafish was first used as a model organism for research in 1937 and have since emerged as a valuable vertebrate system with high physiologic and genetic similarity to humans. One of the earliest uses for zebrafish was as a tool for the toxicology field, specifically as a model to study the liver and hepatocarcinogenic effects of toxins.
The use of zebrafish as a vertebrate screening model for preclinical testing is increasing owing to the number of advantages and remarkable similarities with mammals. They possess many attractive characteristics for being used in Drug Discovery, such as small size, transparency, gene and protein similarity with mammals (more than 80%), and ease of genetic modification to establish human disease models. The ability to perform gene knockdown, knockout and overexpression facilitates the investigation of mechanisms of action, and zebrafish are commonly used to model human diseases using genetic modifications, most notably CRISPR technology.
The zebrafish embryo is an alternative test model that combines the benefits of an in vivo model with the advantages of an in vitro model, such as a high sample size, with statistically significant results. The high fecundity (200 to 300 offspring per couple of adult fish per week) and fast organogenesis of zebrafish allow a High Content Screening (HCS) that would be difficult to achieve in rodents as they are time-consuming and cannot be studied at a large scale.
Also, the use of zebrafish is in line with the 3Rs (Reducement, Refinement, and Replacement) of animals approach for scientific purposes by replacing higher-order animals with lower-order ones, particularly, zebrafish embryos or larvae below 120 hours post-fertilization (hpf) considered as non-protected stages under the European legislation on laboratory animals (EU Directive 2010/63).
The main difference between the mammalian and zebrafish liver is the structural organization of the liver tissue. Despite the anatomical differences between the species, cells constituting the liver are the same as in mammals and a functional liver is present in the zebrafish embryo by 72 hpf, with Phase I and II metabolic enzymes already present by 120 hpf, which is therefore a suitable time point to start hepatotoxicity assays in this NAM.
Mechanistic understanding of Drug metabolism in humans compared to test species is essential for the translation of effects between species. The metabolism of compounds in humans is mainly performed by Cytochrome P450 (CYP) enzymes, which are predominantly localized in the liver. Zebrafish metabolize drugs using similar pathways to those in humans. Most of the zebrafish CYP genes are direct orthologs of human CYPs, enabling metabolic reactions including hydroxylation, conjugation, oxidation, demethylation, and de-ethylation. Goldstone and colleagues identified 94 CYP genes in the zebrafish genome and based on homologous amino-acid sequences, they reported these genes fitted into 18 CYP gene families, that are also present in humans and other mammals. The most important CYP in humans responsible for catalyzing the majority of known Drug-metabolizing reactions, CYP3A4, has an ortholog in the zebrafish, named, cyp3a65. These similarities between humans and zebrafish support the potential of the zebrafish as an animal model for DILI.
A wide variety of methods can be used to assess liver toxicity in zebrafish, starting from visual assessment of gross and microscopic morphological changes, to serum enzyme and biomarker tests, hepatic excretory tests, or assessment of alterations in chemical constituents of the liver. Usually, 3 to 5 days post-fertilization (dpf) larvae are treated with the drugs for 48 to 72 hours. Following exposure to a range of potentially hepatotoxic Drugs, the zebrafish liver develops histological patterns of injury compared to those of the mammalian liver. Biomarkers for liver injury can be also quantified in the zebrafish circulation. Gene expression studies with a set of reference hepatotoxicants in zebrafish embryos resulted in the identification of proteome markers for the detection of adverse responses in the liver, notably Igf2bp1, Cox5ba, Ahnak, Itih3b.2, Psma6b, Srsf3a, Ces2b, Ces2a, Tdo2b, and Anxa1c. Metabolic experiments demonstrate that Drugs are metabolized when exposed to zebrafish embryos by similar reactions to those in humans.
Hepatotox Assay
The mechanisms that occur during the process of liver development in zebrafish have been characterized, leading to a piece of important knowledge about the signaling pathways that govern liver function. These studies reveal that liver precursors cells are already present by 48 hpf and are actively detoxifying at 5 dpf.
At Biobide, due to the complexity of the DILI, we have developed several hepatotoxicity assays (Hepatotox Assays) for the prediction of hepatotoxic agents through different endpoints. Using them complementarily will allow a better prediction of the DILI, and various mechanisms of action, such as acute hepatic toxicity, steatosis, or hepatomegaly.
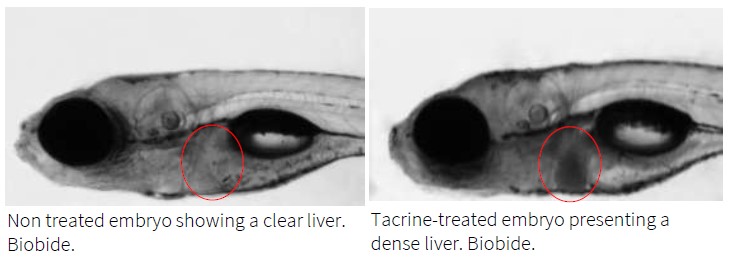
The acute hepatic toxicity assay is a rapid assay that can help to classify potential hepatotoxic compounds, in a fast screening in a pluri-cellular and multi-organ context in a short period. Moreover, the concentration that is inducing toxicity can be measured by bioavailability assays. Acute hepatic toxicity of compounds can be detected through liver opacity assessment in wild-type and transgenic zebrafish, or through changes in liver fluorescence intensity and size in transgenic zebrafish line.
The following images show acute hepatic toxicity assessment by liver opacity:
Zebrafish larvae are amenable to morphological assessments of hepatotoxic effects of compounds using transgenic reporter lines, which can demonstrate specific liver pathologies such as hepatomegaly and fibrosis. They can be complemented with other analyses such as expression levels of genes related to hepatic metabolism (rt-qPCR quantification) or liver alteration assessment through histopathology.
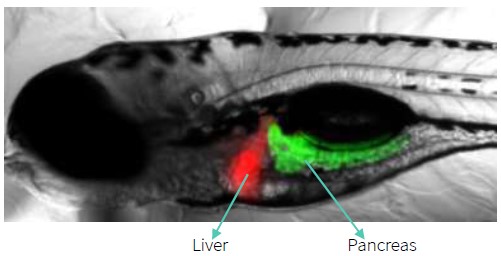
Biobide has a transgenic zebrafish line with liver-specific fluorescent and even a double transgenic line with red fluorescent in the liver and green fluorescent expression in the pancreas. Since fluorescence in the organs greatly facilitates and fastens observation, those transgenic lines are useful tools for the study of hepatotoxicity applicable to High Content Screenings.
Therefore, Biobide’s Hepatotox Assays are a robust screening tool for the analysis of hepatotoxicity that can apply to High Content Screening of DILI.
Sources
- https://pubmed.ncbi.nlm.nih.gov/30312655/
- https://pubmed.ncbi.nlm.nih.gov/31069672/
- https://pubmed.ncbi.nlm.nih.gov/35868597/
- https://pubmed.ncbi.nlm.nih.gov/34832899/
- https://pubmed.ncbi.nlm.nih.gov/33666192/
- https://pubmed.ncbi.nlm.nih.gov/33239838/
- https://pubmed.ncbi.nlm.nih.gov/24773296/
- https://pubmed.ncbi.nlm.nih.gov/23128142/
- https://pubmed.ncbi.nlm.nih.gov/25663337/
- https://pubmed.ncbi.nlm.nih.gov/24626481/
- https://biobide.com/portfolio/hepatotox-assay





