Around 36 million people in the European Union live with rare diseases such as Dravet Syndrome, previously known as Severe Myoclonic Epilepsy of Infancy (SMEI). Dravet Syndrome is a rare type of epilepsy caused by a genetic disorder that triggers, difficult to control, seizures in children. The first symptoms usually manifest in the first year of life, when children may experience an assortment of types of seizures, such as focal, myoclonic and/or facial, atypical absence seizures or generalized tonic clinic seizures.
This syndrome was first described by Charlotte Dravet, a French psychiatrist and epileptologist in 1978; she based her career studying child epilepsy and intellectual disability. It is estimated that from every 15,700 to 40,000 live births 1 will be affected with Dravet. Antiseizure therapy is required in order to treat patients, to decrease the extent and number of seizures. Today’s treatment options have an overall limited effectiveness, due to the fact that the treatment is only symptomatic, and drug resistance is a typical characteristic in these types of treatments. Therefore, the development of new treatments and investigation of the causes and the molecular mechanisms underlying the disease is a priority. In this sense, the Early Drug Discovery process and basic research play a crucial role and both need appropriate animal models to screen new compound efficacy and to generate appropriate disease models to look into the pathophysiology of this syndrome. Thus, New Alternative Models (NAMs) that permit faster, cheaper, and more ethical animal research are of great interest. Among them, the zebrafish model is emerging as a reliable, fast, and cost-effective alternative.
What is Dravet Syndrome?
It was not until 2001 that it was discovered that the cause of Dravet Syndrome was genetic. Genetic alterations in the sodium voltage-gated channel α subunit 1 gene (SNC1A) were observed, classifying this pathology as a genetic epilepsy syndrome and a developmental and epileptic encephalopathy.
Approximately 70-80% of the analyzed patients present genetic alterations in the SCN1A gene including mutations, chromosomal rearrangements, duplications, and exonic deletions.
Then there is a scarcer number of patients, around 20-30% that do not have pathogenic variants in SCN1A, these patients still remain without a molecular diagnosis, which suggests that probably other genes, physiological conditions, and/or environmental factors are involved in the generation of the disease.
In order to find these, further genes have been studied and identified in patients with the pathology and some of them were found to be of importance such as PCDH19, SCN1B, GABRA1, STXBP1, CHD2, SCN2A, among others. For example, pathogenic variants of PCDH19 have been identified in about 5% of patients with Dravet Syndrome.
Current Treatment for Dravet Syndrome
As there is no cure for Dravet Syndrome, today’s treatment goals are to diminish the number and length of the seizures, avoid status epilepticus, endorse better neurocognitive development and, overall, improve patients´ quality of life.
According to the 2022 International consensus on the diagnosis and management of Dravet Syndrome, the maintenance and antiseizure therapies options were classified by the panel of experts as:
- First line of treatment: Valproate
- Second line of treatment: Stiripentol, Fenfluramine, and Clobazam
- Third line of treatment:
- Fourth line of treatment: Topiramate and Ketogenic diet.
Antiseizure treatments have a well-known limitation, that patients in the course will highly end up creating drug resistance. This is why the need of research for new treatment alternatives and the causes and the molecular basis of the disease is imperative. The tedious process that a new Drug has to undergo in order to get regulatory approval and the mind-boggling costs are the main obstacles to delivering new drugs into the market. For this reason, the use of NAMs in the pre-clinical stage, such as the zebrafish model, can speed up and give reliable data in the Early Durg Discovery and development process. Furthermore, the use of appropriate animal models of the syndrome is necessary to better understand the pathophysiology of the disease.
The use of Zebrafish in Dravet Syndrome studies
Zebrafish, Danio rerio, is an Indian small fish that present several benefits. It small size, fast organogenesis, high productivity, and transparency of embryos make them an ideal model for High Content Screening (HCS) assays. Furthermore, regarding the ethical aspect, in Europe, zebrafish are not under animal care policies until they can feed by themselves and around by 5-6 days post fertilization (dpf). A vast array of assays are performed in this embryonic/larval stage making this model in compliance with the 3Rs Principles (Replacement, Reduction, and Refinement), and a time and cost-effective model.
Besides, this model presents a high genetic homology with humans (>80% of the human's genes related to diseases are maintained in the zebrafish) together with their feasibility for genetic modification, allowing the creation of diseases models; the aforementioned transparency and the presence of the blood-brain barrier, highly resembling human neurophysiology. All these features offer important scientific benefits and enable highly reliable information and direct visualization of internal body structures, making it an excellent NAM.
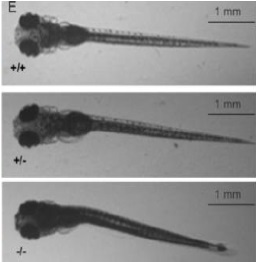
Biobide is a pioneer in using the zebrafish model in preclinical studies and has vast experience in many areas such as rare diseases. For the preclinical efficacy studies of Dravet Syndrome Biobide uses the Zebrafish didys552 line harboring a mutation in the scn1lab gene, the ortholog of human SCN1A and SCN1B. This homozygous mutation is conserved across zebrafish, mice, rats, and humans. The didys552 line phenotypically endures spontaneous electrographic seizures from 3 dpf, allowing for toxicity and efficacy screening of compounds in a HCS manner and basic research of the disease on zebrafish embryos.
Conclusions
Dravet Syndrome is a rare and complex genetic disease that affects children from a very early age and that disturbs importantly their quality of life. Today’s treatment options in a short term of time create tolerance and only tackle the symptoms, not the origin. There is a real necessity to bring to market new treatment options for those suffering from this rare syndrome. The Drug Discovery and Development process becomes more and more expensive every day and regulatory authorities become stricter. The usefulness of NAMs such as zebrafish is becoming very relevant, as represents a cost-effective alternative that offers reliable results rapidly and in line with animal care concerns. Biobide is making a huge effort collaborating with several rare disease foundations and Non-Governmental Organizations, generating gene editing techniques and acquiring (if already generated) and characterizing rare disease models in zebrafish to help the research and the Drug Discovery process in this area to improve the therapeutic alternatives of these devastating diseases.
Sources
- Inicio | Fundación Síndrome de Dravet (dravetfoundation.eu)
- Rare diseases (europa.eu)
- Dravet syndrome: Management and prognosis - UpToDate (unav.es)
- Dravet syndrome: Genetics, clinical features, and diagnosis - UpToDate (unav.es)
- https://pubmed.ncbi.nlm.nih.gov/35490361/.
- https://pubmed.ncbi.nlm.nih.gov/27264139/





