Animal models are essential for the discovery of mechanisms and treatments for neuropsychiatric disorders, including anxiety. Anxiety disorders are one of the most prevalent mental disorders and are associated with significant comorbidity and morbidity.
A typically observed behavior related to anxiety disorders is the avoidance and reduction of exploration, and, therefore, it is an endpoint widely used in animal models for anxiety studies.
In this sense, thigmotaxis or “wall hugging” is a well-validated index of anxiety in animals and humans that measures the reduction of exploratory behavior, and is one of the most commonly used behavioral endpoints measured in pre-clinical studies using rodent models. Likewise, it has been shown that the zebrafish animal model can be used for this behavioral study, even in their larval stage below 6 days post-fertilization.
The use of the zebrafish presents several advantages compared to other animal preclinical studies (such as rodents) in the study of anxiety, including cost-effectiveness, less time consumption, and easy implementation of high-content screenings.
Furthermore, the use of larvae fish is an alternative model that fits the 3Rs principles, in line with the ethical concerns related to the use of animal models studies.
.png?width=509&height=318&name=R%20(1).png)
What is anxiety?
Anxiety is a brain state induced by exposure to threatening or dangerous stressors that persists even after the removal of the trigger, in anticipation of future threats, leading to behavioral changes such as an enhancement of defensive behavior.
Based on epidemiological data, anxiety disorders are the most prevalent mental disorders around the world, and estimations suggest a current global prevalence of anxiety disorders of 7.3%.
Mental health disorders are highly complex and heterogeneous, comprising multiple interacting mechanisms. Rather than tackling multidimensional problems, it may be helpful to focus on individual quantifiable features to simplify such disorders in diverse model systems. The endophenotypic approach is an attractive solution, wherein complex disorders are dissected into measurable behaviors that can be compared across organisms. Thus, avoidance and the reduction of exploratory behavior are typically observed in anxiety disorders and can be used as quantifiable features through the thigmotaxis tests.
Thigmotaxis behavioral model
Thigmotaxis is a well-validated index of anxiety in animals and humans and is one of the most commonly used behavioral endpoints measured in preclinical studies using rodent models. It is characterized by a reduction in exploratory behavior and avoidance to enter the unfamiliar open space. Animals that present a higher state of anxiety show a thigmotaxic behavior strongly avoiding the center of an arena and staying or moving close to the borders of a novel environment.
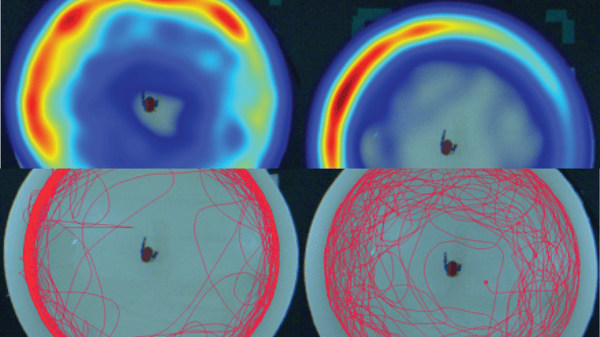
This behavior seems to be caused by the fear of exposure to predators in open spaces. In consequence, animals prefer to stay close to the perimeter of the space available, in order to minimize exposure to threats., This behavior is evolutionarily conserved by a wide range of species, including rodents, fish, and humans.
According to these premises, anxiolytic drugs, such as diazepam, have been found to reduce thigmotaxis while anxiogenic compounds, including caffeine, increase thigmotaxis.
Several studies show that humans with high anxiety sensitivity and agoraphobia also have a higher tendency to keep to the edges of the arena in open-field tests, while their healthy counterparts spent more time in the center of the arena. Analysis of thigmotaxis in rodent models has been demonstrated to serve as a good model for the study of anxiety disorders in humans. Likewise, the zebrafish thigmotaxis model represents a modified version of the open-field test used in rodents and humans, that has been successfully validated, even in the larval stage of the zebrafish.
Anxiety studies in Zebrafish
The zebrafish animal model has become popular due to several advantages it has, including its small size, fast growth, high reproductivity, and high genetic homology to humans (70-75%). In addition, one of the most interesting features this model has is the possibility of using the animals in their larval stage, until 6 days post-fertilization. This feature represents a critical advantage of not using adult animals that are ethically protected and under the supervision of animal care commissions. Besides, larval zebrafish present different advantages compared to other animal models including fast complete organogenesis in 4 days post-fertilization, transparency that allow a direct visualization in vivo, and small size allowing scalable assays with multi-well plates that suppose a huge time and cost efficiency.
Specifically, zebrafish is a popular model for studying neurological phenomena and have been used extensively to model psychiatric disorders and to identify pharmacological interventions and toxicity of drugs and chemical compounds. Several behavioral assays have been developed to quantify neuropsychiatric phenomena in larval zebrafish.
Zebrafish show conserved physiological and neuronal pathways and many neurobehavioral parallels have been uncovered with mammals, including humans. Although there are substantial differences between zebrafish and human brains, studies indicate the molecular mechanism regulating anxiety are similar in both species. Both the human and zebrafish hypothalamic-pituitary-adrenal (HPA) axes, also known as the hypothalamic-pituitary-interrenal (HPI) axis in zebrafish, are activated in response to stress, leading to the production of cortisol. Unlike in rodent models, where corticosterone is the main stress hormone, cortisol is the main stress hormone in both fish and humans. Depression and anxiety, amongst other mental health disorders, have been associated with HPA anomalies and cortisol dysregulation. Hence, the zebrafish shares a core feature of human stress biology making this model especially interesting for these types of studies.
In addition to the general benefits of the use of larval zebrafish, they are particularly well suited for behavioral studies because of their relative maturity in terms of swimming capacity and functionality of the motor, sensory, and stress-regulating systems, and ability to perform simple motor tasks and perceive relevant cues from the environment. Also, by 4–5 days post-fertilization, zebrafish larvae inflate their swim bladder and start to exhibit a broad range of behaviors, including hunting, avoidance, escape, phototaxis, and thigmotaxis that can be readily quantified in automated assays. Thus, many behavioral tests can be carried out in larval zebrafish as early as the initial 6 days post-fertilization.
As mentioned above, thigmotaxis relies on the physical exploration of the spatial zones of a given environment. It is quantified as a percentage of the distance moved in the outer zone of the well out of the total distance moved. Zebrafish are a diurnal species regulated by homeostatic and circadian mechanisms. It has been demonstrated that locomotor activity can be enhanced by exposing larvae to a challenge known as the visual motor response test, consisting of a sudden transition from light to total darkness. In the light-dark transition test, larvae display an immediate and robust increase in locomotor activity upon exposure to darkness. This response also results in enhanced exploration of the physical environment compared to baseline conditions. These findings suggest that dark environments are aversive to zebrafish larvae and this could be used to prompt the expression of anxiety-like behaviors such as thigmotaxis.
The larval zebrafish model has been validated for thigmotaxis assays as replicates the pattern seen in adult animals and other models such as rodents in response to a challenge like illumination changes. Therefore, a version of the open field test can be used as a measure of anxiety levels.
In concordance with these facts, thigmotaxis assays on larval zebrafish based on the visual response to light intensity changes have been validated, and actually, they are used to test the effect of different neuroactive compounds. In this sense, the utility of this assay can be exemplified by the use of high-content toxicology screening protocols, such as the thigmotaxis assay developed by Biobide with zebrafish larvae. This assay is highly automated, with treatment delivery by a pipetting robot, the movement of the larvae recorded by specialized equipment prepared to analyze zebrafish behavior (DanioVision, Noldus), and a customized analysis program that compares the thigmotaxic behavior of each larva. This way Biobide can analyze the potential neurotoxic effect of different compounds and drugs in a high-content manner, maximizing the cost-effectiveness and the number of compounds that can be analyzed.
In conclusion, thigmotaxis can be assayed in zebrafish larvae in a high-throughput manner, enabling both toxicologic analyses of chemical compounds and drugs and mechanistic dissection for discovering treatments for mental health disorders, in an ethical, easier, and cost-effective way.